A few years ago, Steven Deeks, MD ’90, was sitting at the back of a large lecture hall when the scientist on the stage mentioned his name. The researcher, speaking about the design of a new cancer immunotherapy, mentioned that Deeks had run one of the first trials. This came as a surprise to Deeks, who is an internationally recognized expert on HIV – not cancer. However, he learned that his decade-old study had paved the way for a new means of turning the immune system against tumors.
“It was the first time, and certainly not the last time, that I realized all this progress in other areas of medicine traces back to progress we made with HIV,” says Deeks.
The impact goes well beyond cancer. The enormous amount of funding and intellectual capital spent on HIV research has led to many advances in science and health care – from clinical trials to infectious diseases to organ transplants.
At UC San Francisco, the ripple effects of HIV – the human immunodeficiency virus – are especially clear. In 1983, UCSF faculty members opened the doors to Ward 86, the country’s first HIV clinic, at San Francisco General Hospital (SFGH). Over the four decades since, hundreds of physicians, nurses, social workers, and researchers have worked in Ward 86, across UCSF, and around the world to pioneer new ways of preventing and treating HIV. Today, many of them are using what they learned to help patients facing different diseases.
“With HIV, we had a problem that inspired a lot of people, and we had a lot of funding,” says Deeks. “When you bring all these different stakeholders together and give them the right resources, you really start to make a difference in medicine.”
When Community Voices Shape Clinical Trials
On July 1, 1981, Paul Volberding, MD, began his first day of work at SFGH, where he intended to start a new cancer program. But within days of Volberding’s arrival, the wards had an unusual influx of patients. Young, gay men were coming in covered in purple blotches and rashes – Kaposi’s sarcoma, a skin cancer usually only seen in older adults. As their disease progressed, these once-healthy men often developed other mysterious problems: diarrhea, blindness, constant sweating.
It would be months before their underlying disease was given a name – acquired immunodeficiency syndrome, or AIDS – and more than two years before the underlying virus, HIV, was discovered. In those early years, scientists weren’t sure exactly how the virus spread. Across the country, some doctors even refused to treat AIDS patients because of concerns about catching their disease.
“When we started taking care of AIDS patients at the start of the epidemic, they were young people with serious and fatal complications that were just incredibly difficult to manage,” says Volberding. “In many cases, part of the challenge was that [the patients didn’t have] any kind of traditional family support.”
Many of the men Volberding treated were his age, and he felt driven to do whatever he could to help them. Just two months into his new job, he co-founded the nation’s first Kaposi’s sarcoma clinic at UCSF Medical Center. By the time AIDS was recognized by the U.S. Centers for Disease Control, Volberding had immersed himself in research on the disease. He went on to help open Ward 86 and eventually became the co-director of the UCSF-Gladstone Center for AIDS Research. But he also became deeply involved in community organizations around San Francisco.
With few treatment options available beyond pain relief, Volberding worked to make patients comfortable, investigate what might be causing their disease, and start testing new interventions. In 1982, before HIV had even been discovered, Volberding began a trial of an immune drug to treat the Kaposi’s sarcoma associated with AIDS. But to carry out these efforts, clinicians like him had to collaborate closely with the gay community, which was rallying behind HIV patients and providing a range of assistance.
“Without really planning it, a lot of physicians at San Francisco General found ourselves working side by side with community organizations,” Volberding says.
As these support networks grew, academic researchers developing the first clinical trials for HIV maintained close ties with local organizations and family doctors, who were often treating patients after they left the hospital. For the first time, researchers were getting community input as they planned their research programs and clinical trials.
“We wanted to make sure the things that academic physicians were interested in studying were the same things that the community felt were important,” says Volberding. “That input ended up being really critical.”
Men with HIV, for instance, asked Volberding and his colleagues to turn more attention to preventing some of the secondary infections – like salmonella and tuberculosis – that often ravaged their bodies. Until that request, Volberding says, most researchers had been focused almost entirely on HIV itself. At the same time, the scientific community learned how to gain the trust of men likely to contract HIV, how to enroll them in trials, and how to design trials in an ethical way.
Today, many of the ethical principles that the HIV community shaped are used in every area of medicine.”

At the time, the only way for most men with HIV to receive any meaningful treatment was to enroll in clinical trials. But the way most trials had long been run meant excluding men who had other complicating conditions and assigning many patients to control groups that did not receive the new treatment. To maximize the number of HIV/AIDS patients who could receive experimental drugs, doctors and ethicists worked together to design trials differently. For example, some compared their results to previously collected data rather than to a control group, enabling more patients to access the experimental therapy.
“All of that seems commonplace now, but HIV really broke new ground when it came to the way researchers interact with community organizations and the way clinical trials are designed,” Volberding says. “Today, many of the ethical principles that the HIV community shaped are used in every area of medicine.”
The Race to Understand Immunity
By the 1990s, doctors knew the basics of how HIV was causing disease (by attacking the body’s immune cells) and how the virus spread (through bodily fluids). New infection rates in North America and Europe were falling in the wake of public health campaigns. Yet patients already infected with HIV still struggled. By 1994, AIDS had become the leading cause of death for Americans between the ages of 25 and 44.
Deeks, who began caring for people with HIV during this time, recalls when he and his colleagues had just one drug to try to slow the progression of HIV. Called azidothymidine, or AZT, it blocked the ability of HIV-infected cells to make new copies of the virus. For a short time, the drug lowered virus levels in infected people. But HIV evolved quickly, eventually developing resistance to the drug in most patients. Other options were urgently needed.
“We knew we had to do something, so we started really pushing the envelope, trying whatever we could think of,” says Deeks, who is now a professor of infectious diseases at UCSF.
He and other scientists pursued a variety of ideas. However, developing better treatments required a massive investment in basic research. In the 1990s, that investment happened; federal funding for HIV/AIDS increased from millions of dollars per year to billions, hitting $10 billion annually by 1999. Researchers needed to understand exactly how the immune system was reacting to HIV and the details of its full life cycle. Thanks to the influx of attention and funding, many immunologists began devoting their labs to these questions.
“HIV blows up the whole immune system, which is terrible for patients but turns out to be really interesting for scientists,” says Deeks. “You can essentially use this virus to see what happens when the immune system is shut down in a targeted way, and then what happens when you treat it.”
These experiments revealed a complex crosstalk between dozens of different immune cells and molecules – information that formed the bedrock of new immunology knowledge. It became the basis for many other studies on viruses, antiviral drugs, and vaccine development.
Eventually, researchers discovered that antiviral drug combinations targeting many different parts of the HIV life cycle could send HIV patients into long-term remission – successfully shifting the disease from a fatal diagnosis to a chronic but manageable condition.

Over the last couple of decades, the science pursued in the fight against HIV has proven valuable to many other efforts to improve health, particularly immunology. Although trial after trial of HIV vaccines has failed, the lessons learned in each attempt – including exactly how the human body produces antibodies and how to control those pathways – made the development of vaccines against other diseases easier.
“Even though we have not yet been successful at an HIV vaccine, the HIV community has gotten really good at tracking what a vaccine is doing in the body and whether it is working,” says Susan Buchbinder, MD, a professor of epidemiology and biostatistics at UCSF and the director of HIV prevention research at the San Francisco Department of Public Health.
For example, a new vaccine against respiratory syncytial virus – approved for infants in 2023 – was possible largely because of knowledge gleaned from HIV research about how to target viral proteins. Similarly, when COVID-19 emerged, scientists quickly turned to methods for studying SARS-CoV-2 that had long been used to understand HIV and develop potential vaccines against the virus.
A Model for Beating Pandemics
In 2020, many longtime HIV researchers found themselves reminiscing about 1981. A new virus was once again circling the globe. This time, though, they had decades more experience in identifying, understanding, and fighting viral illnesses. At many institutions, including UCSF, the first scientists and clinicians to steer their efforts toward the emerging COVID-19 pandemic were those who had focused their careers on HIV.
The infrastructure and expertise built to counter HIV laid the groundwork for a speedy response to COVID-19. Compared to the years it took to identify and isolate HIV, SARS-CoV-2 was isolated – and its genome sequenced – in just weeks.
“The whole reason the scientific community was able to come up with a vaccine for COVID-19 in less than a year was because of the backbone of HIV research,” says Peter Hunt, MD, a UCSF professor of experimental medicine. “The way the vaccine was designed, the way we sequenced the virus, the way we measured the immune response to the virus and developed tests to quantify viral loads – it all leads back to HIV.”
The innovations spurred by the rush to conquer HIV also led to Deeks’ surprising connection to cancer. In the ’90s, a small group of investigators developed a new way to genetically alter the immune system’s T cells, letting them control what these cells recognized and attacked. They approached Deeks about trying to engineer these chimeric antigen receptor (CAR) T cells so they would destroy any HIV-infected cells in a person’s body. Deeks readily agreed and launched one of the earliest clinical trials of a CAR T-cell therapy.
“It almost worked,” recalls Deeks. “We showed that these cells could effectively bind to HIV and that they could be used safely.”
Years later, researchers realized that adding another immune molecule to the mix made the CAR T cells far more powerful. Building on Deeks’ first trial, they found the therapy very effectively targeted cancer cells. Today, several CAR T-cell therapies have been approved by the U.S. Food and Drug Administration, and more than 30,000 patients with blood cancers in the U.S. have been treated with the therapy. It’s now considered one of the most promising new ways to attack cancer, and researchers at UCSF and elsewhere are racing to extend its benefits to other areas of medicine.
A Blueprint for Hepatitis
Every Friday morning, a brightly painted van parks on a street corner in the Tenderloin district of San Francisco and opens its doors. Inside, staff members ready their supplies to screen people for hepatitis C, a virus that infects the liver and can lead to long-term disease and death. The team hopes to attract patients who are most at risk for the disease, including people using injectable drugs or experiencing homelessness. Rather than wait for these people to visit a clinic, they bring care to them.
The mobile van, dubbed DeLIVER Care, was the brainchild of liver specialist Jennifer Price, MD, PhD, a professor of gastroenterology at UCSF. Price says she took her inspiration from community initiatives to test for and treat HIV.
“The HIV community had been incredibly successful at reaching people who are less likely to interface with the traditional health care system,” says Price. “We had a really good lesson, right here in San Francisco, for how to do that.”
Launched in 2019, the van aims to make more people aware of their hepatitis C status and provide them with treatment. An estimated 2.6% of all adults in San Francisco are positive for the infection, putting them at risk of liver cancer and liver failure. But many people don’t know they are positive.
Like HIV, hepatitis B and hepatitis C are viruses that spread through bodily fluids, cause chronic infections, and weaken the body over time. In the case of HIV, the virus attacks the immune system, while the hepatitis viruses target the liver. Many immunologists study both HIV and the hepatitis viruses because of the similarities in how they work and because about 10% of all people with HIV also live with one or both hepatitis viruses.
In fact, the viruses are so similar that several of the major HIV antiviral drugs also help control hepatitis B. Today, those drugs are used commonly in people infected with both viruses. In addition, the paradigms for testing and treating hepatitis are based on years of fine-tuning the protocols for HIV. Those years led to two takeaways: Test everyone and treat early.
For the first 20 years of the HIV epidemic in the United States, physicians recommended that only people with risk factors for the virus get tested regularly. But those risk factors mostly revolved around people’s sexual behavior and injectable drug use – matters they might not be comfortable disclosing to a physician. The recommendation meant that people who didn’t share this information, or didn’t fit the risk profile, typically wouldn’t be tested.
Annie Luetkemeyer, MD, a UCSF infectious disease physician, recalls a patient she treated many years ago who was being seen for severe headaches. A scan showed an area of damaged tissue in her brain, and doctors carried out an invasive biopsy to try to find the cause, suspecting a tumor.
“It ended up that she had toxoplasmosis, which is a classic infection associated with HIV,” recalls Luetkemeyer, who was a trainee at the time. “Sure enough, she tested positive. If we had known her HIV status up front, we could have avoided the brain biopsy. But she was in a demographic where HIV didn’t cross anyone’s mind.”
Because of stories like that, in 2006, the U.S. Centers for Disease Control and Prevention recommended routine HIV screening for all patients aged 13 to 64. In 2020, similar recommendations were issued for hepatitis C.
“It’s the same story with hepatitis C,” says Luetkemeyer. “There are so many people now diagnosed that don’t know how they got it and don’t have classic risk factors. But with hepatitis, I think we got to this place of universal testing more quickly because of HIV.”
Liver specialist Jennifer Price delivers care via a van to people with hepatitis C, an idea inspired by HIV community outreach efforts. Photo: Elena Zhukova

Infectious disease physician Annie Luetkemeyer says HIV’s test-and-treat model is helping thwart hepatitis C. Photo: Elena Zhukova
HIV insights also led to guidance to treat everyone with a hepatitis infection – even those without symptoms – and to begin treatment as soon as someone tests positive, without requiring a follow-up appointment. For years, clinicians worried that such an approach would lead to problems for patients. But studies on HIV showed that this tactic ultimately led to more patients receiving care than would have otherwise been the case.
In 2023, Price confirmed that this approach works for hepatitis C: 92% of people who received same-day treatment had undetectable amounts of virus in their blood at a later appointment. It was the first U.S. evaluation of a test-and-treat model for hepatitis C in a neighborhood setting.
“It’s completely accurate to say we adopted the model that had been spearheaded for HIV,” says Price. “And now it’s helping us get rid of hepatitis C.”
An Opportunity to Reshape Global Health
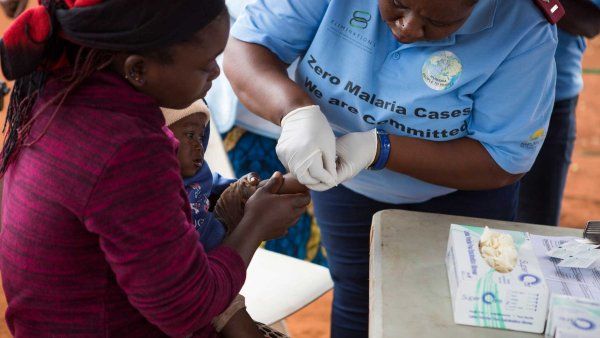
In most of rural East Africa, visiting a health care clinic was long seen as a last resort. Someone could go years without seeing a doctor, traveling to a clinic only for emergency care after a farming injury or because their family was sick with malaria. The idea of regular checkups or preventive medicine was foreign; most clinics were not equipped to follow patients over time or to prescribe medicines that would be taken for more than a few weeks.
In the late 1980s, that began to change. Countries like Uganda – where more than one in seven people tested positive for HIV by 1987 – organized new national health care clinics, trained community workers, and collaborated with funding agencies to support long-term treatment for HIV/AIDS patients. People who had rarely interacted with clinicians were suddenly being followed for years.
“In our country, we take for granted that there is an infrastructure for chronic health care,” says Diane Havlir, MD, UCSF’s Weiss Professor and chief of HIV, infectious diseases, and global medicine at Zuckerberg San Francisco General Hospital. “But before HIV, that was not true in most of the developing world, where there was no such thing as the lifelong treatment of any chronic disease.”

Havlir realized about a decade ago that the public health infrastructure established in Africa to combat HIV could have major benefits for other areas of medicine. In partnership with local communities, she and her colleagues at SEARCH (Sustainable East Africa Research in Community Health) started testing the idea of integrating hypertension and diabetes screening and treatment into the HIV programs they were already working with in Uganda and Kenya. Havlir suspected that if these programs began advertising broad, preventive health care – rather than only HIV treatment – more patients would be apt to use them.
“For so long, HIV care was very siloed,” says Havlir, noting that people didn’t necessarily want to be seen walking into an HIV/AIDS clinic. “When you look at it from the patient perspective, it actually created some barriers and stigma.”
When the expansion first launched in one rural Ugandan village in 2011, Havlir’s group not only diagnosed many new cases of HIV but also hundreds of cases of malaria, tuberculosis, diabetes, and hypertension. Over time, the team found that they could manage chronic diseases over the long term, helping patients get their blood pressure and blood sugar under control. In 2022, they estimated their additional cost of diagnosing and treating hypertension was only about $11 a patient per year – hundreds less per patient than at other health centers in East Africa.
“We’re able to leverage the existing HIV infrastructure in these places to deliver scalable, sustainable health care for many other conditions,” she says. “When we first started trying to tackle HIV in the developing world, it was seen as a really bold move. But now, the benefits have multiplied beyond everybody’s expectations.”