
In the next 30 years, the global landscape of infectious diseases likely will look radically different. Infections that plagued the world for centuries are now on the verge of eradication, or nearly so. Others, however, threaten to disrupt human lives and economies more than ever before. Read on for a glimpse into the future of five of humanity’s most infamous foes: superbugs, pandemic flu, malaria, HIV/AIDS, and tuberculosis.

Superbugs and pandemic flu could kill millions of people by 2050. What will it take to stop these scourges?
Superbugs
Before the advent of antibiotics in the early 20th century, you could die from an infected scrape – if smallpox, cholera, pneumonia, or any other rampant infectious disease didn’t kill you first. It’s no wonder that the average life expectancy back then was less than 50 years.
Today, so many routine medical procedures depend on antibiotics that it is impossible to envision the modern world without them. And yet a global crisis of antimicrobial resistance (AMR) is forcing us to imagine just that. It’s an arms race between deadly pathogens and the drugs that fight them. “And if you had to pick whether the bugs or the drugs are winning, a lot of people would say it’s the bugs,” says Lisa Winston, MD, a professor of medicine at UCSF and an epidemiologist at Zuckerberg San Francisco General Hospital.
If you had to pick whether the bugs or the drugs are winning, a lot of people would say it’s the bugs.”
According to the World Health Organization, these so-called superbugs are “one of the biggest threats to global health, food security, and development today.” Without urgent global action, experts warn, superbugs could kill 10 million people a year by 2050 – more than the current toll from cancer and diabetes combined.
The impact will be felt most acutely in health care settings. “It’s going to start in hospitals because that’s where all of the nastiest bugs are and where there is the most evolutionary pressure” for microbes to develop drug resistance, says pharmaceutical chemist Ian Seiple, PhD, an assistant professor at UCSF’s Cardiovascular Research Institute. “All of the signs are there that this is going to be a really, really big problem.”
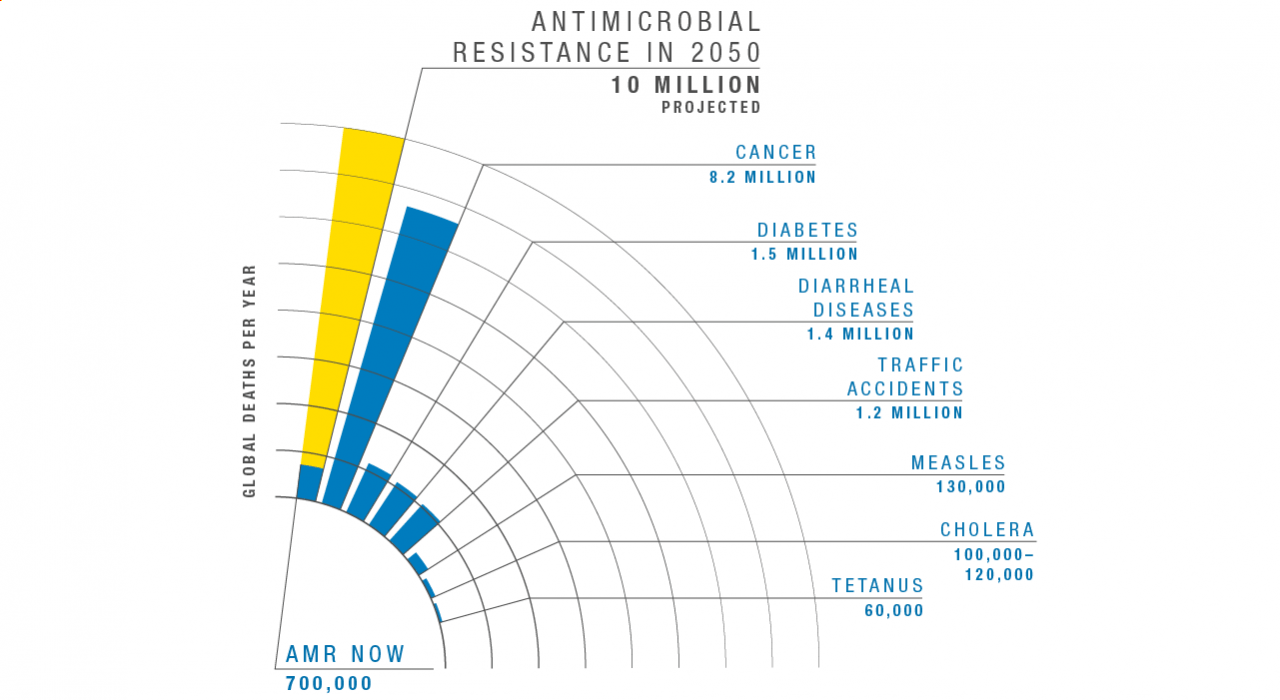
Graph adapted from the Review on Antimicrobial Resistance, comparing deaths by cause in 2016 to projected deaths from AMR in 2050.
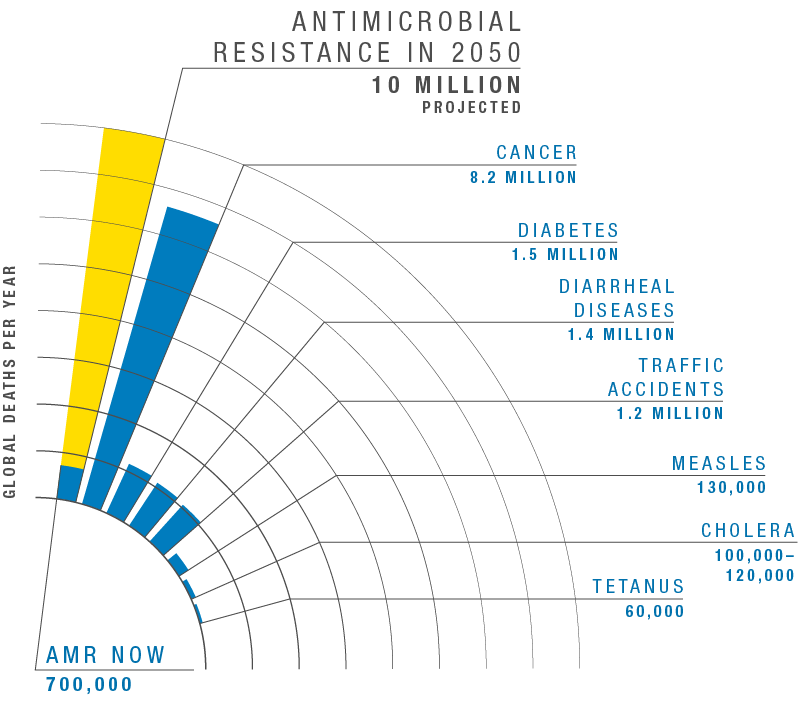
Graph adapted from the Review on Antimicrobial Resistance, comparing deaths by cause in 2016 to projected deaths from AMR in 2050.
Three Strategies for Squashing Superbugs
Reducing Antibiotic Overuse
UCSF is one of many hospitals nationwide that have established antimicrobial stewardship programs to reduce unnecessary antibiotic use. As of September 2019, federal regulations require all U.S. hospitals to institute such programs.
Employing Novel Technologies
The UCSF Center for Next-Gen Precision Diagnostics, led by Charles Chiu, MD, PhD, is among the first labs in the world to utilize advanced genetic-sequencing techniques to quickly identify new infectious threats. Another technology, cryo-electron microscopy, allows researchers to see the precise mechanisms by which resistant organisms evade their attackers. With these tools, scientists could conceivably engineer new antimicrobial drugs fast enough to stay ahead of microbial resistance.
Investing in Drug Discovery
Historically, the world has relied on pharmaceutical companies to capitalize on research breakthroughs in order to bring new antimicrobial drugs to market, but the industry has almost entirely abandoned this effort. Antimicrobial drugs, which cost a fortune to develop but are relatively low-cost and prescribed only in short courses, just aren’t profitable. Ultimately, acquiring a drug arsenal capable of defeating superbugs will require political will and public investment.
Pandemic Flu
“Just like people in California await the next big earthquake, we in the infectious disease field are waiting for the next influenza outbreak,” says UCSF professor of medicine and infectious disease specialist Peter Chin-Hong, MD.
Influenza, commonly known as the flu, is a viral infection of the human respiratory tract. Seasonal virus strains spread around the world annually, doing minimal harm to the average healthy adult. But a few times a century, a new strain jumps to humans from another animal species, such as chickens or pigs, and can trigger a pandemic.
Such cross-species outbreaks are especially contagious and deadly because they can introduce traits against which humans have no defense. Previous flu pandemics have resulted in millions of deaths worldwide. But there’s reason to think we may be able to thwart the next one, says Charles Chiu, MD, PhD, director of the UCSF Center for Next-Gen Precision Diagnostics. Here are a couple of promising approaches:
Improving Surveillance
Vigilant, real-time surveillance and reporting are today’s best hope for stopping an influenza outbreak, Chiu says. His team uses rapid DNA sequencing to identify and diagnose cross-species diseases in remote regions of the world in order to understand, track, and – hopefully – prevent another pandemic.
Pursuing a Universal Vaccine
Seasonal flu vaccines are ineffective against an influenza pandemic. But a vaccine that can fight off any flu – even novel, cross-species strains – might be within reach, Chiu says. “If a universal vaccine becomes available,” he says, “mass global vaccination efforts could nearly or completely control influenza by 2050, just as was done for poliovirus more than 50 years ago.”

Scientists have made incredible strides toward eradicating Malaria, HIV/AIDS, and Tuberculosis. Can we wipe them out for good?
Malaria
Malaria has ravaged humanity for tens of thousands of years. Called the “king of diseases” in ancient texts from India, this wily foe long stymied scientists. Even its name is a misnomer: The Romans thought swamp fumes caused the illness, so they dubbed it mal aria, or “bad air.”
In the 19th century, researchers finally discovered the real culprit: a microscopic parasite called Plasmodium, which is spread by female mosquitoes who inhabit wet, marshy places. Attempts to eradicate malaria gained steam after World War II but then waned in the 1970s and ’80s in the face of enormous challenges, according to Sir Richard Feachem, DSc(Med), PhD, director of the Global Health Group at UCSF. But today the tide is turning again. In the past 20 years, cases of malaria and deaths from the disease have been roughly halved worldwide. Now experts are once again asking: Can we rid the planet of it for good?
The answer is a resounding yes. Given the right tools, strategies, and sufficient funding, the world could be malaria-free by 2050, according to a report published by The Lancet Commission on Malaria Eradication in September 2019. (The commission, co-chaired by Feachem, is a joint endeavor between The Lancet and UCSF.)
But there’s a catch. “To achieve this common vision, we simply cannot continue with a business-as-usual approach,” Feachem says. “We must instead challenge ourselves with ambitious targets and the bold action needed to meet them.”
It’s hard to overestimate what a huge achievement it would be for humankind to eradicate malaria, once and for all.”
Graph shows innovations according to how likely they will be successfully developed (vertical axis), when they will be available (horizontal axis), and their relative effect on accelerating eradication efforts (size of circles). Tap the plus sign to open popups with insights from The Lancet Commission co-chair Sir Richard Feachem. (On your phone? Tap the X in the upper righthand corner to return to the graph). Adapted from The Lancet Commission’s report.
HIV/AIDS
An HIV diagnosis in the 1980s was effectively a death sentence. The virus had hit San Francisco hard, and UCSF clinicians endeavored to care for its many victims. But their drive to overcome HIV/AIDS helped transform the disease from fatal to merely chronic. Research at UCSF spurred the development of antiretroviral therapies, and later, PrEP (pre-exposure prophylaxis), a preventive pill.
“We have the tools to stop the epidemic,” says Paul Volberding, MD, director of UCSF’s AIDS Research Institute and the Weiss Memorial Professor. But eradication by mid-century is unlikely, he predicts. Besides the sheer magnitude of the problem (about 40 million people currently live with HIV), stigma presents a formidable hurdle. “We will make a lot of progress,” he says, “but we still have much work to do on the societal and behavioral issues that perpetuate HIV.”
2018
40 million cases of HIV
Tuberculosis
For a curable disease, tuberculosis (TB) still cuts an astonishing swath of destruction across the globe. The U.S. and most other developed countries have wrestled TB mostly into submission through concerted treatment, education, and prevention measures. But countries plagued with overcrowding, poor hygiene, lack of fresh water, and inadequate public health care systems have struggled to contain the pandemic.
A TB vaccine has been available since 1921, but it is not very effective. “We’ve known for a long time that a better vaccine that can prevent infection and progression to disease in those already infected will have the biggest epidemiologic impact,” says Payam Nahid, MD, MPH, a TB expert and professor of medicine. A recent candidate has shown unprecedented promise, stirring excitement among TB scientists. Meanwhile, researchers are working to improve treatments and diagnostic tests. With this confluence of advances, “the sky’s the limit for improving TB outcomes by 2050,” he says, except for one major stumbling block: “The funding is nowhere near where it needs to be.”
2017
10 million new cases of TB

UCSF Magazine
Dive into the future of health in this special issue of UCSF Magazine.